UK scientists edit DNA of human embryos
- Published
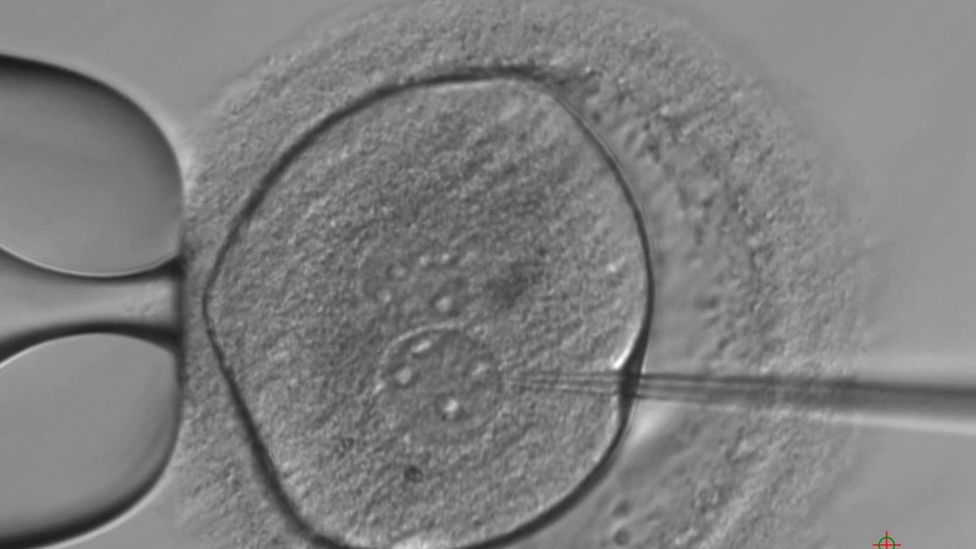
The blueprint for life - DNA - has been altered in human embryos for the first time in the UK.
The team at the Francis Crick Institute are unravelling the mysteries of the earliest moments of life.
Understanding what happens after a sperm fertilises an egg could lead to ways of improving IVF or explain why some women miscarry.
The embryos were modified shortly after fertilisation and allowed to develop for seven days.
The researchers are exploring one of the most astounding of transformations.
We have all journeyed from a single fertilised egg to a human being - built from myriad different tissues ranging from bone to those needed to read this page.
The first few steps on that journey are as critical as they are poorly understood.
Breakthroughs in manipulating DNA have allowed the team at the Crick to turn off a gene - a genetic instruction - suspected to be of vital importance.
The easiest way of working out how something works is to remove it and see what happens.
So the researchers used the gene-editing tool Crispr-Cas9 to scour the billions of letters of genetic code, find their genetic target and break the DNA to effectively disable it.
They were targeting a gene. You are unlikely to have heard of it, but OCT4 is a superstar in early embryo development.
Its complete role is not understood but it acts like an army general issuing commands to keep development on track.
The researchers used 41 embryos that had been donated by couples who no longer needed them for IVF.
After performing the genetic modification, the team could watch how the embryos developed without OCT4.
In a first for UK scientists, human embryos have been genetically modified.
Over the course of the first seven days, a healthy, normal embryo goes from one cell to about 200. It also goes through the first steps of organising itself and handing out specialised jobs to different cells.
The embryo forms a hollow sphere called a blastocyst, with some cells destined to go on to form the placenta, some the yolk sac and others, ultimately, us.
But without OCT4 the blastocyst cannot form. It tries - but implodes in on itself.
From the embryo's perspective it is a disaster but for scientists it has given unprecedented insight.
It is the first time human embryos have been edited to answer questions about fundamental biology.
Dr Kathy Niakan, a group leader at the Crick in London, told the BBC: "When it seemed it was working we were quite excited about the possibility that this would open up.
"This is basic research which is providing us with a foundation of knowledge about early human development."
By deepening understanding of the earliest moments in life, it could help explain what goes wrong in infertility.
During IVF, of 100 fertilised eggs, fewer than 50 reach the blastocyst stage, 25 implant into the womb and only 13 develop beyond three months.
This study alone, published in the journal Nature, cannot explain what is going wrong or why some women miscarry.
But by interrogating all the genes suspected of playing a role in our inception, it could lead to new advances.
Dr Niakan told the BBC: "If we knew the key genes for an embryo to develop successfully that would, I would hope in the future, lead to improvements in IVF technology and give us really important insights into why some pregnancies fail."
One option for IVF is to have a better way of testing which embryos are going to be successful.
Or it may be possible to boost embryos during IVF by growing them in a different culture media - a fertiliser for fertilised eggs.
Ethical debate
These experiments have been legal since 2008 in the UK, where it is possible to manipulate such embryos for 14 days as long as they are not implanted.
But while this application of the technology is answering fundamental questions of science, other research groups are trying to remove genes that cause disease.
That is provoking deep ethical debate.
Dr Sarah Chan, a bioethicist at the University of Edinburgh, told the BBC: "I don't think this study should raise any ethical concerns.
"It is very clear that the aim of the research was basic science and that there was never any intention to create genetically modified human beings.
"That said if we could one day use gene editing in human embryos for medical purposes, the potential benefits could be huge, but before we took such a step we would want to make sure that we'd had a really robust and wide-ranging public dialogue on all of the ethical issues involved."
Dr Rob Buckle, the chief science officer at the UK Medical Research Council, said: "Genome editing technologies are having a game-changing effect on our ability to understand the function of critical human genes.
"As genome editing techniques develop it's vital that this work continues within a robust yet adaptable regulatory framework so that its full potential can be realised in a scientific, ethical and legally rigorous way."
Follow James on Twitter.
- Published13 January 2016