Covid: Are countries under pressure to approve a vaccine?
- Published
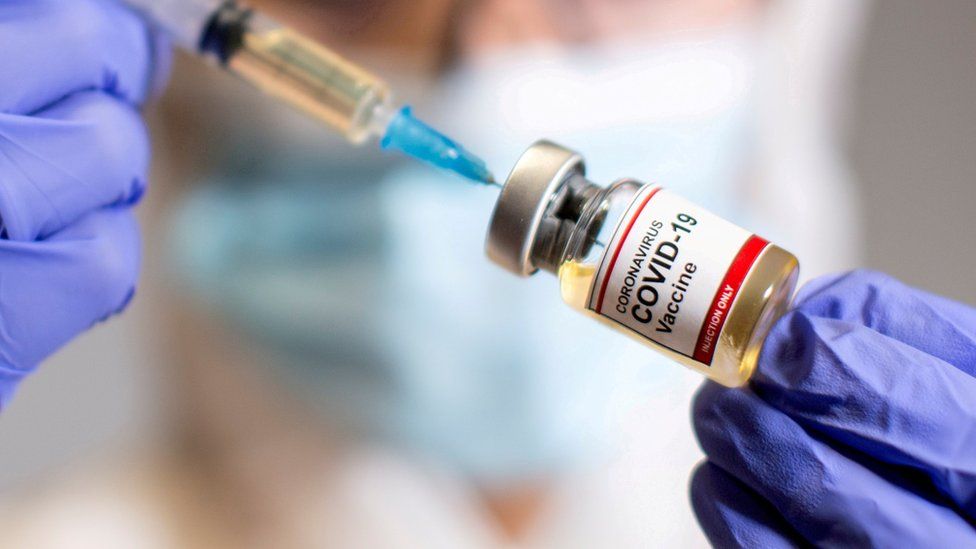
The UK has become the first country in the world to approve the Pfizer/BioNTech coronavirus vaccine, paving the way for mass vaccinations.
The first doses are already on their way to the UK with the first vaccinations penned for next week.
Speaking on BBC Radio 5 live on Thursday, Deputy Chief Medical Officer Jonathan Van Tam said he did not think the US or European regulators would be many days behind the UK.
Approvals elsewhere, he said, were probably "a matter of days" away.
With the UK having given its approval for a vaccine, are nations now under pressure to follow suit?
How have European countries responded?
The European Medicines Agency (EMA), which is in charge of approving the vaccine in the EU, defended its time frame in a statement.
It said it had the "most appropriate" method to approve the vaccine.
Before deciding on whether to approve a vaccine, the EMA studies data from lab studies and large clinical trials.
"These are essential elements to ensure a high level of protection to citizens during the course of a mass vaccination campaign," the statement said.
Under EU law, countries can evoke emergency powers to temporarily approve a vaccine in the event of a pandemic. The UK, still a member of the EMA, was able to approve the vaccine under this rule, despite suggestions from ministers that Brexit had enabled the approval.
Education Secretary Gavin Williamson said on Wednesday that the UK had been able to approve the vaccine because it had "the best regulators".
A European Commission spokesperson hit back, telling reporters: "We are of course absolutely convinced that the regulators in the UK are very good but we are definitely not in the game of comparing regulators across countries nor on commenting on claims as to who is better.
"This is not a football competition. We are talking about the life and the health of people. We have in the EU a very developed system - which by the way still applies to the UK - in order to approve the authorisation of medical products, vaccines and to place them on the market."
The EMA has said it will meet by 29 December at the latest with a vaccine rollout expected within days of that date.
Germany's health minister, Jens Spahn, said despite having the fast-track option, the country had opted to wait for the EMA in order to help boost confidence in the safety of the vaccine.
"The idea is not that we're the first, but the idea is to have safe and effective vaccines in the pandemic and that we can create confidence and nothing is more important than confidence with respect to vaccines," he told a news conference.
"We have member states, including Germany, who could have issued such an emergency authorisation if we'd wanted to. But we decided against this and what we opted for was a common approach to move forward together."
Elsewhere, Russian President Vladimir Putin has ordered the government to simplify procedures for state registration of some medicines, in order to speed up approval of a vaccine.
In August, authorities approved the country's Sputnik V vaccine before Phase 3 trials had even begun. The trial, which involved 40,000 volunteers, has concluded but the result has not been made public.
Mr Putin has told authorities to start immunising people at risk from next week.
'A different path' for Britain
An exasperated sigh sums up the reaction from a number of European capitals to the vaccine victory proclamations of some British government minsters. Gavin Williamson's sweeping assessment that the UK is a "better" country than many of its allies was seen as particularly bold.
One senior diplomat told me he was delighted Britons would soon be receiving the vaccine but that "someone should remind Mr Williamson that the Pfizer/BioNTech vaccine was created by a German company, founded by scientists of Turkish origin, in partnership with an American distributor, and is being manufactured in Belgium before being transported across France to reach the UK".
The claim that Brexit allowed the UK to approve the vaccine faster than other European countries has been disproved but it does reflect once again a different path Britain is taking. All EU countries have the option to follow the UK example and let their domestic drug regulator issue emergency approval, but the bloc says it wants to wait for the European Medicines Agency to give the green light on all their behalf. Germany, backed by Denmark and others, believes this maximises safety, allows a co-ordinated rollout, boosts public trust in the vaccine and ensures no country is left behind.
But some politicians in Poland and Hungary - countries currently at odds with their Western neighbours over emergency Covid funding - have begun to register their discontent. And if the Europe-wide delivery of a vaccine which promises to end the Coronavirus misery for millions is pushed back, there are likely to be more voices asking, "Why can't we have what the Brits have already got?"
What is happening in the US?
Following the news of the UK's approval of the vaccine, the US Food and Drug Administration (FDA) defended its decision to review the data, saying that its scientists reviewed the data more "robustly" than anyone else.
FDA Commissioner Steve Hahn said different groups of scientists were currently looking into the Pfizer data - some were examining safety and others would look at its efficacy.
They will meet on 10 December and share their findings with the advisory board before it is approved.
It is hoped that the vaccine will be rolled out on 15 December.
The FDA has been under pressure from President Donald Trump to act more quickly. He had previously said he wanted a vaccine to be ready before election day.
His reaction to the UK approval is not yet known but US media report that Mr Hahn was summoned to the White House on Tuesday to discuss vaccine approval times.
On Thursday, top infectious disease expert Dr Anthony Fauci said the UK had not scrutinised the Pfizer data "carefully" .
"If you go quickly and you do it superficially, people are not going to want to get vaccinated," he told Fox News.
US government scientist Dr Anthony Fauci says there could be a Covid vaccine in the US before the end of 2020
What about China's vaccines?
There are currently four Chinese vaccines in Phase 3 trials. Some of the advanced vaccines have been approved for emergency use.
Nearly one million people have taken an experimental coronavirus vaccine developed by China National Pharmaceutical Group (Sinopharm), according to the company.
People who have been given jabs include state employees and international students.
In an article posted to WeChat, Sinopharm said no adverse reaction had been reported from those who had taken the experimental vaccine so far.
Hundreds of people queued in Yiwu, China to get an experimental Covid-19 vaccine
Related Topics
- Published15 February 2022
- Published2 December 2020