Did you know you can make fantastic frosted decorations using a salt and water? Find out how in this fun festive experiment. Please note - this activity will require adult supervision!
These Christmassy crystals are made with the power of chemistry! Why not hang them on your tree or create a string of frosty festive shapes? Epsom salt is available at chemists, in the bathing section.
For a full explanation of the science behind crystallisation and written instructions on how to do this activity, follow the link below.

Crystal creation doesn't always go to plan, which can be really frustrating! But fear not, we've put together some advice. To help you with your own efforts, we’ve put together some top crystal science tips with the help of Dr Heather Milton, Royal Society of Chemistry Education Coordinator at the University of Edinburgh.
Make sure your solution is saturated
To create crystals, you need to heat a liquid and mix in a soluble solid (one that dissolves, such as a sugar or salt) until no more of the solid can be dissolved. As more can be dissolved in the hot liquid, when the solution cools, some of the solid re-emerges as crystals.
You won’t see any crystal growth unless your solution is completely saturated. This means that absolutely no more of your chosen solid can be dissolved in the hot water - you need to keep stirring it in until you are getting some left undissolved at the bottom of the liquid. To avoid crystals sticking, you can try pouring your solution into a different container or filtering it using a coffee filter, but this isn’t always necessary (in our experiment, we dug our crystals out with a fork).
We used Epsom salt, but you can use other solids, including table salt or sugar. These will each behave differently – sugar is more soluble than Epsom salt and it will take a greater mass of sugar to saturate the same volume of water.

Our method is designed to work quickly by leaving the solution in the fridge overnight. This quickly cools the solution, making crystals form faster.
While this is great for making nice frosty decorations, if you want to grow chunky crystals then you’re going to have to cool your solution more slowly. This means leaving your solution in a cupboard or cool, dry corner and waiting for a few days (or even weeks) for crystals to take shape. In nature, giant salt crystals form by cooling in caves over thousands of years!
Be mindful of moisture
It’s not just temperature that affects crystal growth, humidity (the moisture of the surrounding air) has an impact too. Crystals grow more easily in dry environments, where solutions are less likely to absorb water vapour.
You may find that crystals don’t grow in your fridge because it's too humid. In this case, you may have to try leaving your solution in a different cool, dry place and waiting for a while longer.

If at first you don't succeed… then try again! If you’re not having much luck after leaving your solution to cool, try putting a pinch of your solid in the bottom of your container. This is a good test of whether your solution has enough solid dissolved in it – if it is properly saturated, this can help to kick-start crystal growth.
If this doesn’t work, it might be worth starting again. Was your solution saturated? Was your chosen spot too humid? Was it possible that the temperature went up, causing any crystals that had formed to dissolve again? Consider all of these variables, make adjustments and keep trying!
If you’re interested in exploring crystal growth further, check out the results of the Royal Society of Chemistry’s 2014 Global Experiment. People all over the world tried growing crystals to see how their local environment affected crystal size and shape.
How do Christmas lights work? interactive
Do you know the science behind how light bulbs function?
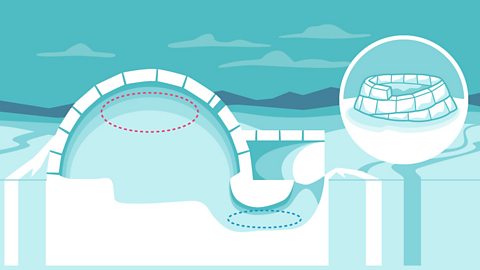
Why doesn't an igloo melt? interactive
How do igloos stay strong and warm? Find out more about the amazing process to build a house out of snow.